Abstract
Introduction:
Salvage chemotherapy followed by high dose therapy with carmustine, etoposide, cytarabine, and melphalan (BEAM) with ASCT is standard treatment for patients with chemosensitive, relapsed/refractory (r/r) NHL. Patients with inadequate response to salvage chemotherapy portend a worse prognosis. We present a phase I dose-escalation trial of venetoclax combined with BEAM conditioning followed by ASCT for the treatment of r/r or high-risk NHL. An expansion cohort of 10 patients was added at the highest tolerated dose.
Venetoclax is an oral BCL-2 inhibitor that has significant single-agent and combination activity in r/r NHL. Preclinical observations showed that venetoclax potentiates the cytotoxicity of chemotherapy, increases synergistic cell kill, and can overcome chemoresistance even in highly aggressive lymphomas. In this trial, we aim to test the safety of the combination of venetoclax plus BEAM followed by ASCT. We hypothesize that venetoclax will help overcome the chemoresistance of these tumors and decrease post-ASCT relapse.
Methods:
This is an open-label, single-center, phase I trial (NCT03583424) with a dose expansion cohort of venetoclax in combination with BEAM and ASCT for patients with r/r or high-risk NHL. Venetoclax is given for 10 days, starting at D-10 before ASCT, in three dosing cohorts (400, 800, 1200 mg) using a quick ramp-up schedule. There were no dose limiting toxicities; therefore an expansion cohort of 10 additional patients accrued at the 1200 mg dose. Eligible participants include adult, fit patients with B and T-cell NHL refractory after upfront induction therapy, in partial remission or progressed after salvage, requiring ≥ 3 lines of therapy, relapsed within 1 year of induction, or at a high risk of relapse after ASCT (in first complete or partial remission). Patients with small lymphocytic lymphomas, with primary, or uncontrolled secondary, CNS lymphoma patients are excluded. Primary outcome is safety and determination of maximal tolerated dose (MTD). Adverse events (AEs) were recorded using CTCAE v 4.1 between days -10 to -6 then the Bearman scale was used from day -6 until engraftment.
Results:
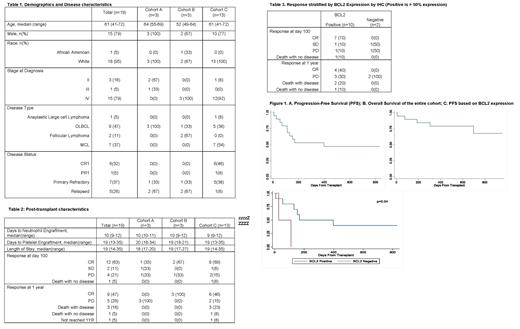
Dose-escalation proceeded with no DLT and 19 patients were accrued across three cohorts. Baseline characteristics and safety data for all three cohorts are presented in Table 1. The majority were male (79%) and had stage IV disease (79%). The media age was 61. No unusual toxicities were encountered beyond what is expected with BEAM. No serious AEs were observed between days -10 to -6 that was related to treatment. No tumor lysis syndrome or other toxicities attributable to venetoclax were observed. Engraftment was as expected. Post-transplant, on the Bearman scale, there were no grade 3 toxicities, 5 grade 2, and 8 grade 1 toxicities. In cohort 3, 1 patient died prior to engraftment from fulminant sepsis. At 100 days post-transplant, 63% were in CR and 11% had stable disease. Fourty-seven percent remained in CR at 1 year post transplant (Table 2). After a median follow-up of 654 (range 335-957) days, median progression-free survival (PFS) was 398 days and median overall survival (OS) was not reached (Figure 1). Six patients went on to receive CD19 chimeric antigen receptor T-cell (CAR-T) therapy. Data of response according to BCL2 expression by IHC is presented in Table 3 for the 12 patients who had available data. Median PFS was superior in BCL2 positive group (p=0.04; figure 1C)
Discussion:
The addition of venetoclax to BEAM appears to be safe and well-tolerated in a typical, elderly population with high-risk disease and produced promising results. While CD19 CAR-T are a consideration, many patients may not have access to these therapies. Venetoclax in combination with BEAM can represent an option for patients with relapsed and refractory NHL.
Brammer: Celgene: Research Funding; Seattle Genetics: Speakers Bureau; Kymera Therapeutics: Consultancy. Maddocks: Celgene: Divested equity in a private or publicly-traded company in the past 24 months; Karyopharm: Divested equity in a private or publicly-traded company in the past 24 months; Beigene: Divested equity in a private or publicly-traded company in the past 24 months; ADC Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months; Morphosys: Divested equity in a private or publicly-traded company in the past 24 months; KITE: Divested equity in a private or publicly-traded company in the past 24 months; Pharmacyclics: Divested equity in a private or publicly-traded company in the past 24 months; BMS: Divested equity in a private or publicly-traded company in the past 24 months; Merck: Divested equity in a private or publicly-traded company in the past 24 months; Novatis: Divested equity in a private or publicly-traded company in the past 24 months; Janssen: Divested equity in a private or publicly-traded company in the past 24 months; Seattle Genetics: Divested equity in a private or publicly-traded company in the past 24 months. Saad: Kadmon: Research Funding; Amgen: Research Funding; careDx: Consultancy; Incyte Pharmaceuticals: Consultancy; Magenta Therapeutics: Consultancy; OrcaBio: Research Funding. Jaglowski: Novartis: Consultancy, Research Funding; CRISPR Therapeutics: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Juno: Consultancy; Takeda: Consultancy. William: Guidepoint Global: Consultancy; Dova Pharmaceuticals: Research Funding; Merck: Research Funding; Kyowa Kirin: Consultancy; Incyte: Research Funding.
Venetoclax is a BCL2 inhibitor approved for treatment of hematological malignancies (AML and CLL).
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal